Maintrac®
How it works
Maintrac®: How it works
IMPORTANT NOTES:
1. Maintrac® is not intended to be used as a diagnostic or screening approach. It may be used for tracking the progression or remission of tumours of epithelial origin through its detection of circulating epithelial tumour cells (CETCs) in the blood. The approach has multiple applications, including helping to inform clinical judgment in the provision of functional or integrative support for patients or clients.
2. If Maintrac® to be used to inform cancer treatment directly, or to monitor the interaction of chemotherapeutic agents with CETCs or CSCs (circulating stem cells), the tests must be overseen by a registered medical practitioner or oncologist.
Maintrac® provides doctors and health professionals with a way to monitor epithelial tumour cells which can help inform collaborative clinical decision-making with patients.
The cells from solid tumours circulating in blood (CETCs) determine the risk of blood-borne metastases. Monitoring the response of these cells can be used to help inform personalised interventions.
Maintrac® testing is based on a simple blood sample. The method quantifies and measures in real-time the circulating epithelial tumour cells (CETCs), in contrast with other methods that rely on preanalytic manipulation of the sample, such as fixation, microfiltration or enrichment techniques. Maintrac®, as a real-time monitoring technique, is highly reproducible and the SIMFO GmbH laboratory in Bayreuth, Germany that conducts the tests is fully accredited according to ISO 15189:. To-date, over 100 peer-reviewed articles on Maintrac® have been published in high quality journals worldwide.
Maintrac® enables the response to different therapeutic measures to be monitored because the number of CETCs in the blood has been shown to be correlated with tumour growth and the development of metastases.
Tumours consist of heterogeneous cell populations with various cell types that may each respond differently to different interventions or therapies. Evidence accumulated over many years using Maintrac® has found that the likelihood of developing metastases increases significantly as a result of rising cell numbers of CETCs released into the patient’s blood by the tumour. This is why it is important to identify, quantify and characterise a patient’s cells in order to help inform optimal therapies and monitor a patient’s progress over time.
Maintrac® can:
- Monitor the effects of single, combination or multi-modal therapies:
Patients whose circulating epithelial blood cells are completely eliminated or considerably reduced will tend to have a reduced risk of relapse. - Personalise therapeutic approaches:
Maintrac® allows for different chemical and natural agents to be tested against the specific cell types of a given patient, thereby supporting the tailoring of targeted therapies for the individual. - Track the effectiveness of hormone therapy:
The risk of relapse is known by oncologists to be reduced if the number of cells in the blood does not rise during hormone therapy, and subsequently may go on to gradually decline. The proliferative activity of CETCs depends on their number of hormone receptors. Hormone receptor blockers such as Tamoxifen block these receptors, preventing cell proliferation. Where primary tumours have been removed, for example in HER2/neu over-expressors who are receiving treatment with Herceptin (trastuzumab), Maintrac®’s ability to detect CETCs can be used as a surrogate test to evaluate HER2/neu status.
REMINDER: Maintrac® is not intended to be used as a diagnostic or screening approach. It may be used for tracking the progression or remission of tumours of epithelial origin through its detection of circulating epithelial tumour cells (CETCs) in the blood. The approach has multiple applications, including helping to inform clinical judgement in the provision of functional or integrative support for patients or clients. If Maintrac to be used to inform cancer treatment directly, or to monitor the interaction of chemotherapeutic agents with CETCs or CSCs (circulating stem cells), the tests must be overseen by a registered medical practitioner or oncologist.
Methodological approach
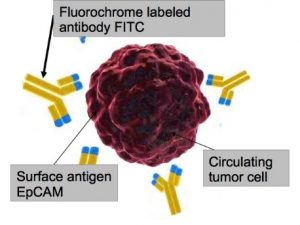
Maintrac relies on being able to measure epithelial cell adhesion molecules, EpCAM for short, that are a type of antigen that is expressed on the epithelium of most solid tumours. These antigens are not usually found on blood cells. This antigen can be stained with a green fluorescent antibody. The live EpCAM-positive cells are detected and enumerated microscopically. This is possible with an additional dead/live staining dye called propidium iodide. All cells that the dye is able to enter due to having a permeable membrane are excluded. Normal epithelial cells are also detected if these are present. However, if the patient has a tumour, it is most likely that the epithelial cells are tumour cells.
PHYSICAL APPEARANCE OF TUMOUR CELLS
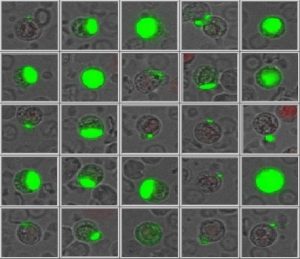
The strength of the EpCAM signal on the cells has a high range of variation. Maintrac® can also detect cells with very low EpCAM expression and these may in some cases be the most significant indicators of metastasis (the epithelial-mesenchymal transition leads to loss of EpCAM).
Accuracy
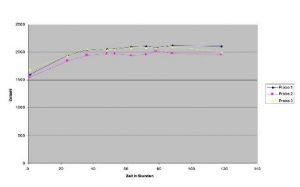
Repeated measurements provide highly comparable results. In the example shown, three parallel samples of the same patient show almost identical results that remain constant over time.
Clinical data provided by Professor Pachman’s Laboratory, Bayreuth, Germany.
Reproducibility
Duplicate assays for more than 80 patients showed excellent correlation.
Clinical data provided by Professor Pachman’s Laboratory, Bayreuth, Germany.
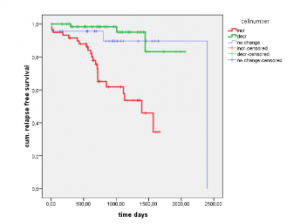
Clinical relevance
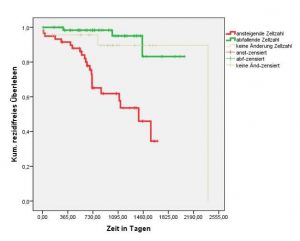
Patients with increasing cell counts during therapy have a higher risk of relapse (red Kaplan-Meier curve) than patients with decreasing cell numbers (green Kaplan-Meier curve).
This allows timely adjustments to therapy.
Clinical data provided by Professor Pachman’s Laboratory, Bayreuth, Germany.
Please note that it is known that tumour cells can downregulate their antigen expression, including the epithelial surface antigen (EpCAM), during increasingly aggressive growth or the so-called epithelial-mesenchymal transition (EMT). This would imply that they may become less detectable or even undetectable for our approach that uses an antibody against EpCAM to detect cancerous cells in blood. In this case we not infrequently observe – after an increase in cell numbers – a sudden decrease to below our detection limit. Under these circumstances we would recommend turning to imaging, and you may wish to consider Stemtrac®, which is used to identify cancer stem cells. We strongly suggest patients remain under the care of their oncologists at all times.
